Extracellular Fluid – Composition And Fluid Flow
Table of Contents
As life begins, water solutions play a key role in chemical reactions. In addition to dissolved substances, a solution also contains solutes. A human body consists of a wide variety of solutes, including proteins – the ones that transport lipids, carbohydrates, and, most importantly, electrolytes. As used in medicine, an electrolyte is mineral-free from the charge carried by a salt (an ion). An electrolyte, such as sodium ions (na+) or chloride ions (cl-), may also contain other ions. In this article we shall discuss Extracellular Fluid in detail.
A process called osmosis occurs in the body in which water moves between compartments by crossing membranes of semi-permeable cells. A semi-permeable membrane allows water to diffuse between regions of higher concentration and regions of lower concentration, by way of an osmotic gradient. Therefore, water will enter and exit cells and tissues depending on how concentrated these solutes and waters are. The balance between the solutes inside and outside of the cells should be maintained to maintain normal function.
Compartments Of Fluid
A tiny blood vessel surrounded by several cells is shown in the diagram. The interstitial fluid (if) is an extracellular fluid (ECF), which is fluid between body cells. This also refers to the fluid in blood vessels. The intracellular fluid (ICF) inside each body cell is the fluid that surrounds it.
There are different body fluid compartments, which are essentially separate from each other due to some physical barrier. Fluid-containing intracellular membranes are located in a compartment called intracellular fluid (ICF).
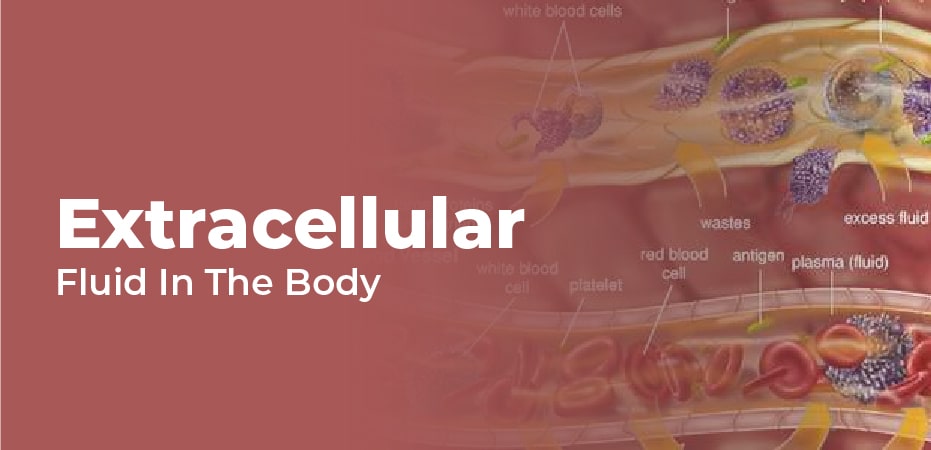
all bodily tissues are covered with extracellular fluid (ECF). It consists of two primary components, the fluid portion of the blood (called plasma) as well as the interstitial fluid (if) in which all non-blood cells reside.
Extracellular Fluid In The Body
The remaining one-third of the body’s amount of water comes from the ECF. Plasma contains roughly 20 percent of the ECF. During 24 hours, plasma transports a wide range of materials including blood cells, proteins (including clotting factors and antibodies), electrolytes, nutrients, gases, and wastes.
The if transports gas, nutrients, and wastes from capillaries to cells. Cell membranes separate the if from the interior of the cell by allowing selective passage of materials.
In addition to ETFs based on water, there are other waters available to the body. There are many types of fluids in the body, including cerebrospinal fluid that bathes the brain and spinal cord, lymph, synovial fluid in joints, pleural fluid, pericardial fluid, and the peritoneal fluid.
In addition to being considered ECF fluids, these fluids are not inside of cells themselves.
Intracellular Fluid
ICFs are a principal component of cytosols and cytoplasms within cells. On average, a male adult generates about 25 liters (seven gallons) of fluid in their icf, which makes up about 60 percent of the total fluid in their body.
Living cells typically have a very high concentration of water, which allows their volume to remain very stable. A cell’s cytosol becomes too concentrated with solutes if the amount of water inside it falls to an inadequate level; if too much water enters the cell, it could burst and die.
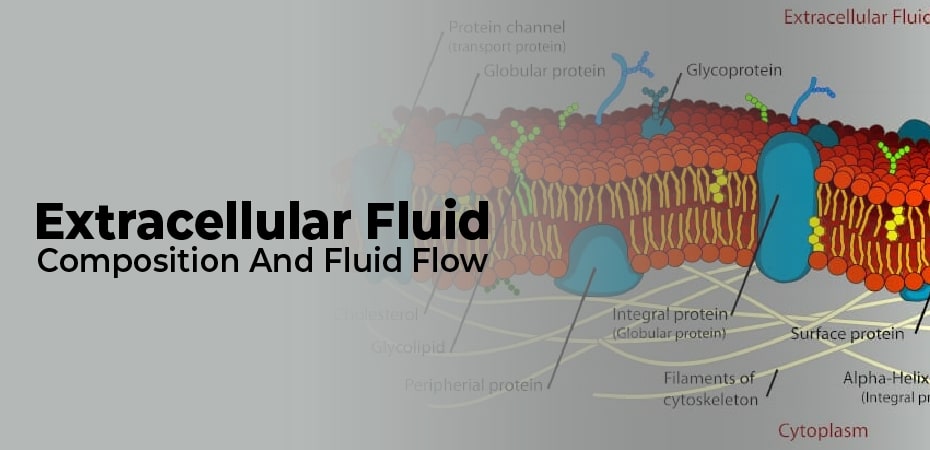
Extracellular Fluid Composition
Anions and cations are the main components of extracellular fluid. Na+ and k+ together represent 136-145 meq/l, potassium (3.5-5.5 meq/l), and calcium (ca2+ = 8.4-10.5 meq/l). Chloride, hydrogen carbonate, and hydroxide are anions (meq/l and 22-26 mm, respectively). Water travels through these ions.
Water makes up 93% of plasma, plus dissolved proteins, clotting factors, na+, ca++, mg++, hco3-cl-, hormones, and carbon dioxide (plasma’s main function is excretory excretion).
Several different physiological processes are mediated by dissolved substances, such as gas exchange, blood circulation, and the treatment of medical conditions in the body.
Fluid Movement Between Compartments
Fluids move between compartments under hydrostatic pressure. During heart pumping, blood exerts hydrostatic pressure against blood vessel walls. During a blood-clotting process, hydrostatic pressure (also known as capillary blood pressure) is higher than “colloid osmotic pressure” in the blood (which is primarily made up of circulating albumin) at the capillary end.
Plasma and nutrients are forced out of capillaries. Caverns receive fluid and waste in tissues at the venule end, where hydrostatic pressure is greater than osmotic pressure. Through the filter, blood plasma is squeezed into the if around the cells.
The lymphatic system reprocesses and cleans surplus fluid in the interstitial space that goes unreturned to the capillaries, and then it enters the veins at the subclavian venous system.
Osmotic gradients also influence fluid movement between compartments. A semipermeable membrane provides a gradient in concentration of all solutes on both sides.
As a function of the concentration difference between the solutes within the cell membrane, the magnitude of the osmotic gradient increases. The solute and water concentrations change when water passes across the osmotic membrane.
Water transfers from plasma to the intracellular fluid (and the reverse) and from the intracellular fluid to the extracellular fluid (and the reverse). A body’s fluid compartments are constantly replenished and drained depending on the conditions in different parts of the body.
Conclusion
Your body consists mostly of water. In body fluids, solutes refer to substances with varying concentrations. Cellular functions depend on water and solute concentrations. Cell functions are impaired because of the loss of water in the cytosol.
If cells ingest water, they may rupture their membranes and burst their cells. It occurs when fluid exerts pressure against a wall and forces fluid to move between compartments. A gradient can osmotically move fluid as well.
The movement of certain solutes between compartments is assisted by ATP. Passive transport occurs when molecules or ions flow easily through membranes, and when pressure gradients exist between high and low concentrations.