How to Find Ionization Energy? Bohr’s Atomic Model
Table of Contents
How to Find Ionization Energy? Finding ionization energy can be a tricky task, but with a little bit of information and some basic calculations, it’s possible to figure out what numbers you need to know.
Ionization energy is the energy needed to break an atom’s nucleus into its constituent protons and neutrons. This process is what creates atoms and molecules.
There are a few ways to find ionization energy. You can use statistical methods or you can use the Pauli exclusion principle.
What Is Ionization Energy?
Ionization energy is the measure of how much energy it takes to remove an electron from an atom. The higher the ionization energy, the more difficult it is to remove an electron from an atom.
This makes sense because atoms with high ionization energies have a strong attraction to their electrons. Ionization energy is important because it helps us understand how atoms interact with each other and form molecules.
Ionization Energy Formula
Chemists use the ionization energy equation to calculate how much energy is needed to remove an electron from a molecule or atom. The equation is as follows:
E = q x V
Where E is the energy in Joules, q is the electric charge of the electron in Coulombs, and V is the voltage in volts. This equation can be rearranged to solve for any of the variables.
Factors Governing Ionization Energy
There are many factors that govern the ionization energy of an atom. The most important of these are the nuclear charge of the atom, the electron configuration of the atom, and the distance between the nucleus and the electron.
Other factors that can affect ionization energy include the presence of other atoms or molecules in close proximity to the atom being ionized and the temperature of the system.
The nuclear charge of an atom is determined by the number of protons in the nucleus. The higher the nuclear charge, the greater the force exerted on electrons by protons in the nucleus and therefore, the higher the ionization energy.
The electron configuration of an atom is also important in determining its ionization energy. Electrons that are closer to the nucleus have a greater attraction to protons and are more difficult to remove than those that are further away from the nucleus.
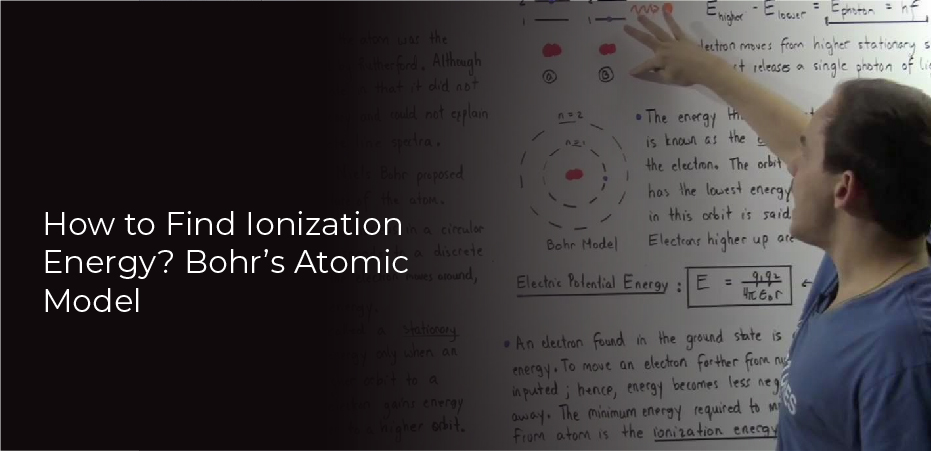
Ionization Energy and Bohr’s Atomic Model
Ionization energy is the amount of energy needed to remove an electron from an atom. The higher the ionization energy, the more difficult it is to remove an electron from an atom. Ionization energy is used to determine the reactivity of atoms and molecules.
The Bohr model is a model of the atom that uses quantum mechanics to describe the movement of electrons in atoms. The Bohr model uses the concept of ionization energy to explain how electrons move around the nucleus.
Ionization Energy Trends in the Periodic Table
The ionization energy of an atom is the energy required to remove one electron from an atom in its gaseous state. The ionization energy of an element increases as you move from left to right across a period and decreases as you move down a group.
This is because the nuclear charge (the positive charge of the nucleus) increases as you go from left to right across a period, while the number of protons in the nucleus (which determines the nuclear charge) remains constant as you go down a group.
The electron shielding, which occurs when the outer electrons surround the nucleus and partially cancel out the nuclear charge, also decreases as you move down a group. This means that it is easier for an electron to be removed from an atom when it is located closer to the nucleus.
How to Determine the Ionization Energy of an Element?
The ionization energy of an atom is the minimum amount of energy required to remove an electron from the atom. This energy is specific to each element and can be used to identify an element by its ionization energy. The higher the ionization energy, the more difficult it is to remove an electron from the atom.
There are a few ways to determine the ionization energy of an element. One way is to look up the ionization energies of each element in a table. Another way is to use a formula that takes into account the electron configuration of an atom.
The most accurate way to determine the ionization energy is through experimentation. Electrons are removed from a sample of atoms one at a time and the amount of energy required for each removal is measured.