If you’re an aspiring student of chemistry or physics, then this blog post is for you! Are you wondering how many electron does carbon have? Carbon takes on a variety of forms, so it’s not easily determined.
In this blog post, we’ll break down the fundamentals of atomic structure and use different theories to gain insight into what exactly holds together the unified elements that make carbon such an imperative part of organic life. Get ready to dive deep into the world of atomic structure – let’s go!
The Role of Electrons in Carbon
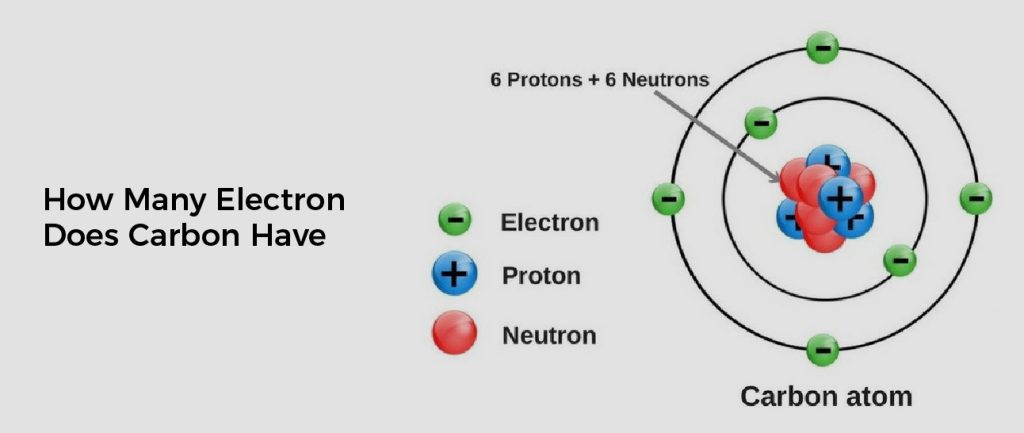
Carbon is a chemical element with an atomic number of 6, which means it has 6 protons and 6 electrons in its outer shell. The two electrons in the outermost energy level are called valence electrons, and they influence how carbon interacts with other elements.
Carbon typically forms 4 bonds with other atoms, which means it has 4 valence electrons to share. This is how carbon can form many compounds and molecules, including the backbone of all known life on Earth.
What Is the Number of Electrons in A Carbon Atom?
The number of electrons in a carbon atom is 6: two in the inner shell and four in the outer shell. Carbon has an atomic number of 6, meaning it has 6 protons and 6 electrons. These are 6 electrons; two are in the first electron shell, two are in the second electron shell, and four are located in the third electron shell. These 4 electrons in the outermost shell are valence electrons, which determine how carbon interacts with other elements. Carbon typically forms 4 bonds with other atoms, so it has 4 valence electrons to share.
What Are the Different Forms of Carbon?
Carbon is a very versatile element, and it exists in multiple forms, each with its own unique properties. The most common form of carbon is graphite, which consists of flat layers of tightly bonded carbon atoms arranged in hexagonal lattices.
Diamonds are formed from the same arrangement of carbon atoms as graphite but are held together much more tightly due to the strong covalent bonds between the atoms. Other forms of carbon include fullerenes, nanotubes, and amorphous carbon, which are used in various applications such as batteries and electronics.
Arrangement of Carbon
The number of electrons in an atom plays an important role in the formation of chemical bonds. Whether you’re dealing with carbon or one of the other elements in the periodic table, the atomic number tells you the total number of electrons present in an atom.
Carbon atoms have a small atomic structure but possess a big amount of energy when in an excited state. They can also form covalent bonds, a type of bond in which two atoms share a pair of electrons to form a single bond.
The number of valence electrons in an atom can be easily determined by the following method:
First, subtract the atomic number from the group number for the element on the periodic table. In this case, carbon is found in Group 4A and has an atomic number of 6. The difference between these two numbers is -2, which means that carbon has four valence electrons.
All elements with a negative value will have four valence electrons, and all elements with a positive value will have 5 or 6 valence electrons. Therefore, carbon has 4 valence electrons and can form bonds easily with other atoms.
How Many Valences of Electron Does Nitrogen/ Fluorine & More Have
1. Valence Electron of Nitrogen
Nitrogen has a valence electron of 5, which is the number of electrons that participate in the formation of bonds and are shown as the outermost shell of an atom.
Valence is a mathematical expression that indicates how well an atom can combine with other chemical species. It also shows how many electrons an atom can gain or lose. In some cases, the valency may change due to changes in oxidation.
A valence electron is also called an octave electron. They participate in chemical bonding by forming covalent and polar bonds.
2. Valence Electron of Fluorine
Fluorine is the lightest halogen, and it is also the most reactive. It is a highly toxic pale yellow diatomic gas that reacts with all other elements except light inert gases. It is in group 17 of the periodic table
Fluorine has 7 valence electrons. The valence of fluorine is more than the valence of nitrogen. Carbon is less electronegative than fluorine. As a result, carbon forms covalent bonds by sharing electrons.
3. Valence Electron of Oxygen & Iodine
Oxygen has 6 valence electrons, which are distributed among the molecule’s atoms. Chlorine has 7 valence electrons. Each atom shares electrons with the central atom.
Iodine has 5 valence electrons. This number is more than the valence of nitrogen, which has a valence of one.
4. Valence Electron of Beryllium
Beryllium, also known as Be, is an alkaline earth metal with4 protons, 2 electrons, and 2 neutrons. It is used in various materials, including non-sparking tools, alloys with other metals, and nuclear weapons casings. In addition to being a light metal, beryllium is a strong and brittle element with a very high melting point.
The valence electrons of beryllium are s-type orbitals. They are in the last ring of the atom. These valence electrons are involved in bond formation.
5. Valence Electron of Neon
Neon is a colorless noble gas that is part of the second period of the periodic table. It has a very inert electron configuration. Unlike Sulfur, Neon does not need to gain electrons. The octet rule states that 8 valence electrons are required for stability.
The atomic number of neon is 10. In addition, it has a complete outer shell. Using the Bohr Model of Neon, it has 10 protons, 10 neutrons, and 2 electron shells. One of the 2 electron shells is the L-shell.
The L-shell is a type of outer shell that contains 8 valence electrons. Generally, all elements have these valence electrons. However, some of them have higher energy electron configurations.