How to Calculate Specific Heat Capacity
Table of Contents
There are a number of online calculators that can help you calculate the specific heat of a substance. These tools are available for free and are easy to use. Once you have entered your values and methods, the calculator will compute the amount of heat energy in the substance. It will also tell you the initial and final temperatures. This calculator can be useful in a number of situations.
Method toCalculate Specific Heat Capacity
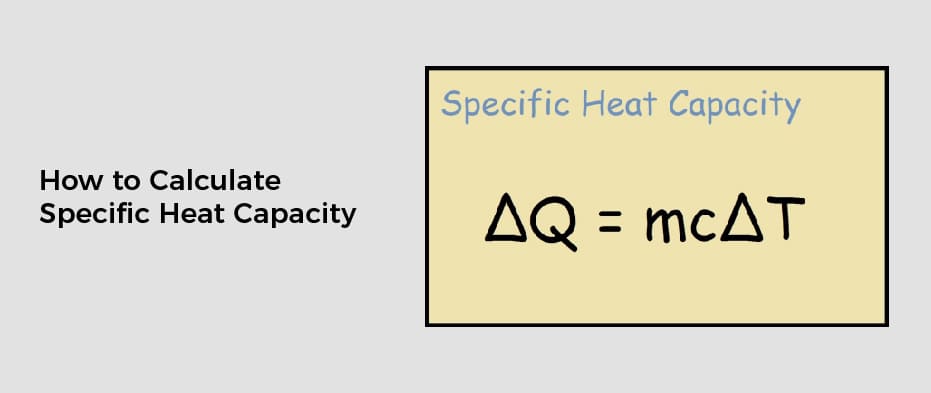
A specific heat capacity is a unit that describes how much heat an object needs to increase in temperature. It is expressed in J kg-1 K-1 and is a measurable physical property. There are many ways to calculate specific heat capacity. One way is to use the rule of mixtures to determine the specific heat of a mixture.
This formula uses the mass and volume of each component to calculate the specific heat. While it is important to note that the units of mass and volume are unequal, the values should match.
This calculator is useful when you need to determine the heat capacity of a sample. This quantity is related to its mass and the amount of energy needed to raise the temperature by one degree, Kelvin. You can also use the specific heat capacity calculator to determine how much heat a sample has. The formula is based on the equation q=mca+t.
HERE ARE THE EXAMPLE OF THE SPECIFIC HEAT CAPACITY OF WATER & IRON
- Water
The heat capacity of Wateris high because of its hydrogen bonds. When water is heated, the hydrogen bonds break and release much energy. That’s why melting 1g of ice at zero degrees requires approximately 334 joules of energy. This is also known as latent heat of melting. Using a specific heat calculator, you can easily calculate the joules of energy required to melt a multiple-gram sample of any substance.
- Iron
The specific heat capacity of IRONis 0.449 J/g*℃, while that of water is 4.18 J/g*℃. The lower the specific heat capacity of iron is, the less heat the iron absorbs. Calculating the specific heat capacity of a material is essential in understanding heat transfer.
The Difference Between Heat & Temperature
Temperature and heat are often used interchangeably in everyday language, but they indicate two very different concepts. Temperature measures the average kinetic energy of molecules in an object; the higher the temperature, the faster the particles move. Heat, on the other hand, is the total energy transferred from one object to another due to a temperature difference. It is measured in joules, the same unit used for energy.
Heat is always transferred from a hotter object to a colder one, and the transfer of energy across the temperature gradient makes thermodynamics possible. Heat can be transferred in three ways: conduction, convection, and radiation. Conduction occurs when two objects with different temperatures are in contact with each other; heat flows from the warmer object to the cooler one until equilibrium is reached. Convection is a convective transfer of heat through fluids, while radiation occurs when energy is emitted from an object in the form of electromagnetic waves.
Overall, temperature and heat are related but distinct concepts in thermodynamics. Temperature is simply a measure of the average kinetic energy in a substance, while heat is the energy transferred between two objects due to a temperature difference. Thermodynamics uses concepts and their associated properties to explain how heat moves throughout the environment.
How To Calculate Specific Heat of a Substance
The specific heat capacity of a substance is the quantity of heat that a substance can hold, given an energy change. This capacity depends on three variables: the mass of the substance, the amount of heat transferred, and the temperature change. This property is measured in joules per gram ℃.
One way to calculate the specific heat of a substance is to place a substance with temperature in a calorimeter filled with 600 g of water. After some time, the system reaches equilibrium. The water absorbs the heat gained or lost by the substance, so the system must be thermally insulated. In the specific heat equation, the gray rectangle’s area represents the substance’s density.
Specific heat capacity is an important concept in thermodynamics, andit tells us a lot about a substance and is unique to each. Specific heat is used in heating and cooling systems, radiators, and the like. For example, copper has a specific heat of 0.385.
Conclusion
In conclusion, temperature and heat are two important concepts in thermodynamics. Temperature measures the average kinetic energy of molecules in an object, while heat transfers energy between two objects due to a difference in temperature. Heat can be transferred in three ways: conduction, convection, and radiation. Thermodynamics uses these concepts to explain how energy moves through the environment.