What Are Isotopes in Chemistry
Table of Contents
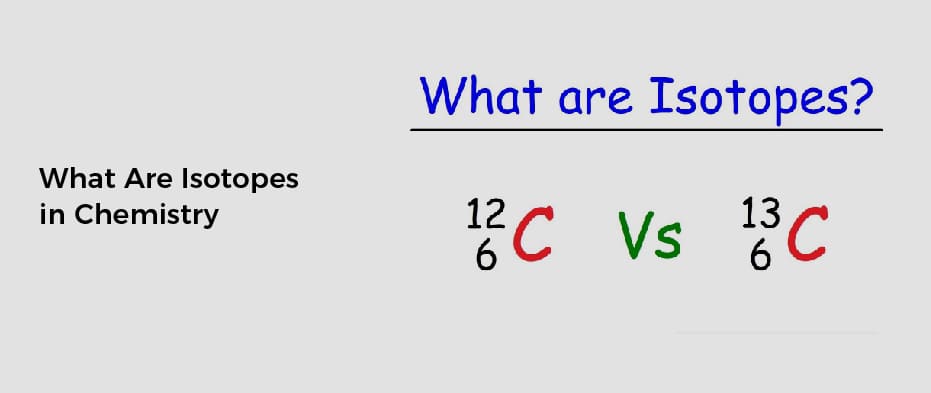
Atoms can have two different types, known as isotopes. Although isotopes have the same atomic number, they differ in their nucleon number because of the different numbers of neutrons in their nuclei. These differences are important when studying the structure of the elements and their properties.
Isotopes can be further subdivided into species. For example, there are about 300 stableIsotopes of hydrogen (protium), which can be divided into seven species: protium (H) with a mass number of 1, deuterium (D) with 2, tritium (T) with 3, helium-three (He-III) with 4, helium-four (He-IV) with 5, lithium-seven (Li-VII) with 6 and beryllium-ten (Be-XI) with 9.
What Is an Isotope?
Isotopes are atomic nuclei with the same number of protons but different neutrons. Isotopes differ from each other by having different numbers of neutrons. The difference in their number of neutrons means they have different atomic masses. For example, an isotope with 1 neutron is called a proton (proton), while an isotope with 2 neutrons is called a neutron (neutron). Isotopes are used to measure the relative abundance of elements in rocks and minerals.
History and Origin of Isotopes
At the time, this phenomenon was something of a mystery. Many experts believed that it might be caused by some “radiant energy,” which could only be observed indirectly rather than an actual emission of particles and energy. However, scientists like Wilhelm Conrad Röntgen and Marie Curie later discovered that radiation came from individual atoms rather than being a general property of an element.
In 1913, Frederick Soddy discovered that isotopes were different forms of the same elements, which explained how certain atoms could emit radiation while others didn’t. His research also showed a relationship between radioactive decay and chemical changes within atoms – in other words, unstable isotopes would be more likely to decay into different elements.
The Important Factors of Isotopes
· Atomic Number
The atomic number of an element is determined by the number of nucleons present in the nucleus. Two or more different isotopes of the same element have the same atomic number but different nucleon numbers and hence different chemical and physical properties. The atomic number is an important parameter in analyzing the chemical properties of an element.
Atomic mass is another important property of an element. It helps differentiate a given element from another. For example, the atomic number “Z” can be determined from the chemical symbol for the element, ‘C14’. Atomic mass “m” indicates the element is in a metastable state.
In addition to the atomic mass, isotopes are classified according to their relative stability. A stable isotope contains an equal number of protons and neutrons.
· Relative Abundance
The relative abundance of isotopes is an important factor in chemistry. Because the ratios between different isotopes are not identical, it is important to study different types separately. For example, the ratio between stable isotopes and radioactive isotopes is different. This enables scientists to distinguish between stable and radioactive isotopes and calculate the relative abundance of each one.
Relative abundance refers to the number of atoms of each isotope in a sample. The mass of an atom is also known as the relative atomic mass. The relative abundances of isotopes differ by mass, but they are related. This way, you can determine if one isotope is more or less abundant than the other. This difference determines the relative abundance of different isotopes in a sample.
The spectrometer measures the mass-to-charge ratio, which enables scientists to determine the relative abundance of different isotopes. In this experiment, a vaporized sample of chlorine is passed into an ionizing chamber with high-energy electrons. The ionized ions are then passed through a magnetic field. The heavier ions deflect more than, the lighter ones, so the relative abundance of the two isotopes is determined.
· Kinetic Fractionation
Kinetic fractionation is a reaction where one or more isotopes are converted into a different element. This process is usually done using a chemical reaction. There are two ways to do this. First, a reaction can be kinetically initiated. The reaction can be kinetically initiated by mixing one or more isotopes and kinetically stopped by quenching the reaction mixture before it completes.
The rate of kinetic fractionation depends on the number of isotopes. For example, the relative amount of species 2 compared to the initial material isotopically enriched. This relationship is known as the kinetic isotope effect. It is often exponential, and high conversion rates will lead to large changes in isotopic composition.
Many factors can influence the rate of isotope fractionation. In one study, researchers found that the rate of d15N in basidiomycetes was positively correlated with biomass. The kinetic isotope exchange model could explain this, as basidiomycetes preferentially incorporate 14N in their biomass. Moreover, differences in average d15N of basidiomycetes could be explained by the amount of N used during harvest.
Chemical Properties Of Isotopes
Isotopes of an element differ in their chemical and physical properties depending on the number of valence electrons and atomic mass. For instance, hydrogen has a mass of one, while deuterium has a mass of two. Tritium has an atomic mass of three. While each isotope has similar properties, there are subtle differences in its physical properties. For example, they have different activation energies. The difference in activation energy means that they have different reactions.
The chemical properties of isotopes differ from one another because different varieties of an element have different amounts of neutrons. For example, the atomic number of hydrogen is one, but three of its nuclei contain zero, one, and two neutrons. These three elements are called isotopes because they share the same place on the periodic table.
In addition to atomic mass, isotopes differ in chemical and physical properties. For example, the weight of a heavy hydrogen isotope is significantly smaller than that of its lighter counterpart. This makes the heavy isotopes more stable, whereas the light ones are more unstable.
Verdict
How do we know which isotopes are radioactive? What evidence is there that some heavier atoms can decay into lighter ones? How do we measure the stability or instability of different elements, and how does this relate to nuclear physics discoveries in the past?
The idea of using an unstable element as a radiation source dates back to 1896 when physicist Antoine Henri Becquerel discovered that some elements emit radiation spontaneously. This radiation is known as alpha and beta radiation and can be measured using a Geiger counter or similar device.