So, you think determining polar molecules in chemistry is a breeze? Well, think again. The world of polar molecules is a complex web of electronegativity, dipole moments, molecular geometry, and more.
Ever wondered why water is polar while carbon dioxide isn’t? Or how to predict if a molecule will be polar or nonpolar? Get ready to unravel the mysteries of molecular polarity and discover the fascinating world of chemistry’s most intriguing forces at play.
Electronegativity and Polar Covalent Bonds
Understanding electronegativity is key to determining whether a covalent bond is polar or nonpolar. Electronegativity is a measure of an atom’s ability to attract shared electrons in a chemical bond. When two atoms with different electronegativities form a covalent bond, the shared electrons are pulled closer to the more electronegative atom. This creates an uneven distribution of electron density, leading to the formation of a polar covalent bond.
In a polar covalent bond, one atom has a slightly negative charge, and the other atom has a slightly positive charge. This charge separation results in a molecule with a positive and a negative end, known as a dipole. On the other hand, when two atoms with similar electronegativities bond, the shared electrons are evenly distributed, resulting in a nonpolar covalent bond.
Dipole Moments in Molecules
To determine the presence of dipole moments in molecules, consider the distribution of charge within the molecular structure. Dipole moments arise when there’s an uneven distribution of electrons, leading to a separation of charges within the molecule. This results in a positive end and a negative end, creating a polar molecule. The magnitude of a dipole moment is determined by the difference in electronegativity between the atoms involved in the bond.
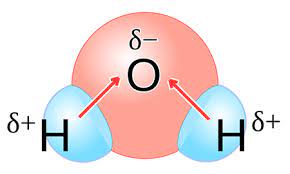
For example, in a molecule like hydrogen chloride (HCl), the chlorine atom, being more electronegative than hydrogen, pulls the shared electrons closer to itself, creating a dipole moment where the chlorine end is partially negative, and the hydrogen end is partially positive.
Understanding dipole moments is crucial in predicting the behavior of molecules in various chemical reactions. Polar molecules tend to interact differently with other molecules compared to non-polar ones due to the presence of these partial charges. By analyzing the dipole moments in a molecule, chemists can gain insights into its physical and chemical properties, aiding in the study and manipulation of substances in the field of chemistry.
Molecular Geometry and Symmetry
When analyzing molecular geometry and symmetry, observe how the arrangement of atoms influences the overall shape of the molecule. The spatial arrangement of atoms around a central atom determines the molecule’s shape. This arrangement is crucial in understanding the three-dimensional structure of a molecule.
The VSEPR (Valence Shell Electron Pair Repulsion) theory helps predict the geometry of molecules by considering the repulsion between electron pairs around the central atom. Different arrangements, such as linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral, result in distinct molecular shapes.
Symmetry elements like rotation, reflection, and inversion play a vital role in determining the overall symmetry of a molecule. Symmetrical molecules have balanced distributions of atoms, while asymmetrical molecules exhibit uneven distributions.
Understanding molecular geometry and symmetry is essential in predicting a molecule’s physical and chemical properties. By examining these aspects, you can gain insights into how the structure of a molecule influences its behavior and interactions with other substances.
Factors Influencing Molecular Polarity
Factors that influence the polarity of a molecule include the electronegativity of its constituent atoms and the molecule’s overall geometry. Electronegativity is the ability of an atom to attract shared electrons towards itself in a chemical bond. When atoms with different electronegativities are bonded, an uneven distribution of electron density occurs, leading to a polar covalent bond. This happens because the more electronegative atom hogs the electrons, creating a partial negative charge on itself and a partial positive charge on the other atom.
Additionally, the overall geometry of a molecule plays a crucial role in determining its polarity. Symmetrical molecules tend to be nonpolar because any opposing polar bonds cancel out due to their arrangement. On the other hand, asymmetrical molecules with polar bonds that don’t cancel out create a net dipole moment, resulting in a polar molecule. Understanding these factors is key to predicting the polarity of different molecules in chemistry.
Examples of Polar and Nonpolar Molecules
Explore the distinction between polar and nonpolar molecules by examining specific examples that illustrate their differences.
Water (H2O) is a classic example of a polar molecule. Due to its bent shape and uneven distribution of electrons, water exhibits a partial negative charge near the oxygen atom and partial positive charges near the hydrogen atoms, making it highly polar.
On the other hand, carbon dioxide (CO2) serves as a prime example of a nonpolar molecule. In CO2, the linear arrangement of the carbon and oxygen atoms results in a symmetrical distribution of electrons, causing the molecule to be nonpolar overall.
Moving on to another example, ammonia (NH3) showcases polar characteristics. The lone pair of electrons on the nitrogen atom contributes to an uneven charge distribution, making ammonia polar.
In contrast, methane (CH4) represents a nonpolar molecule. With a symmetrical tetrahedral shape and equal sharing of electrons, methane lacks any significant charge separation, rendering it nonpolar.
Frequently Asked Questions
When intermolecular forces are present, they can influence a molecule’s overall polarity by either enhancing or reducing it. These forces arise from interactions between molecules and can result in attractions or repulsions.
Yes, a molecule can be nonpolar even if it contains polar bonds. This occurs when the polarities of the bonds cancel each other out due to the molecule’s symmetry or shape, resulting in a net dipole moment of zero.
The shape of a molecule impacts its polarity by determining how the partial charges are distributed. If the molecule’s shape allows for a symmetrical distribution of charges, it may be nonpolar despite containing polar bonds.
Conclusion
So, now you know how to determine polar molecules! By understanding electronegativity, dipole moments, molecular geometry, and other factors, you can easily identify whether a molecule is polar or nonpolar.
Remember, polar molecules have uneven distribution of electrons, leading to partial positive and negative charges.
Keep practicing with examples to solidify your understanding of molecular polarity. Happy molecule hunting!